Which Lewis Dot Diagram Represents a Fluoride Ion
Draw the Lewis structures of C2H6 C2H4 and C2H2. Draw a Lewis electron dot diagram for an atom or a monatomic ion.

Question Video Understanding How To Draw Lewis Structures For Fluoride Ions Nagwa
In almost all Fluorine and neon have seven and eight dots respectively.

. Which Lewis electron-dot diagram represents an atom in the ground state for a the correct Lewis electron-dot structure for the compound magnesium fluorideDec 18 Best Answer. Iodine fluoride has the molecular formula of IF3. Which Lewis electron-dot diagram represents a fluoride ion.
Fluorine is group 17 element in Periodic table. 1 Lewis dot structure of Fluoride ion Valence electrons in F 7 Valence electrons in F- 71 8 thus the lewis dot structure of fluoride ion F- will have 8 electrons around F and a negative charge option e is correct 2 Lewis dot structure of View the full answer. A step-by-step explanation of how to draw the Se Selenium Se2- Selenide ion Lewis Dot StructureFor the Se and Se 2- structure use the periodic table to.
Key term ionnonmetal ions get 8 dots and a negative charge. The allene molecule has the following Lewis structure. What is the lewis dot structure of C2H5F.
Lewis Structure Potassium Fluoride sodium fluoride wikipedia. As we know Hydroxide contains one hydrogen and one oxygen atom. 32 Each diagram below represents the nucleus of.
2 Which ion has the same electron configuration as an atom of HE. Of course the elemental form is bimolecular. Option-A is the correct answer.
Lewis symbol for fluoride ion has 8 dots and a -1 charge. Using Lewis dot diagrams show how some number of atoms of magnesium and atoms of fluorine can transfer electrons to form ions of each element with stable octets. A light ray travels inside a block of sodium fluoride that 1.
To draw fluorine lewis dot structure we have to count valence electrons of Fluorine that is 7 which are written as dots around F. 1 1 3 3. For example fluorine has 7 electrons and so needs only one more electron.
Theres not enough electrons available in the structure for each atom to have an octet by themselves. Fluorine is in Group 17 of the Periodic Table. Which do you think would be bigger.
The number of valence electrons in the atom. Learn how metals react to form ionic compounds and how this effects their properties with BBC Bitesize GCSE Chemistry. Upon reduction the fluorine atom forms fluoride which has 8 valence electrons and is isoelectronic with a Noble Gas which one.
And thus the neutral atom has 7 valence electrons. Fluorine ion lewis dot structure. Fluorine atom or fluoride ion.
Lewis symbol for fluoride ion has 8 dots and a -1 charge. Now in order to draw its lewis structure we first of all calculate the total number of valence electrons of both elements ie. A fluoride ion has the same electronic structure as a neon atom Ne.
The following It gains an electron from another atom in reactions forming a fluoride ion F -. The Lewis dotstructure for iodine fluoride is. H- If so that is the Lewis dot structure for the hydride ion.
A Lewis structure or Lewis dot diagram represents the bonds formed between two. Which Lewis dot diagram represents a fluoride ion. It is harmful by inhalation and ingestion.
It is composedof one iodine I and three fluoride F atoms. What is represented by the dots in a lewis electron dot diagram of an element in period 2 of the periodic table. Draw the lewis structure of Butanal which has the.
Question 16 The Ksp of barium fluoride is 100 x 106The Ksp of calcium fluoride is 390 x 1011. Which Lewis Dot Diagram Represents A Fluoride Ion. Also one negative charge is being carried bu hydroxide ion as OH.
Be sure to include the resulting charges of the ions. Learn vocabulary terms and more with flashcards games and other study tools. How many different elements are represented by the diagrams.
The Lewis Structure electron dot diagram of each ion is used to construct the Examples Lithium fluoride LiF Lithium atom loses one electron to form the. Select the atoms drawn with valid Lewis dot structures. Upon reduction the fluorine atom forms fluoride which has 8 valence electrons and is isoelectronic with a Noble Gas which one.
Which Lewis electron-dot diagram correctly represents a hydroxide ion. It accept electron from donor atom to gain noble gas like stability. Circle the part of your diagram that shows the formation of one formula unit of magnesium fluoride.
Phase diagram is a diagram showing change in the physical state of a substance or a mixture under di. Fluoride-NeonThe Lewis Structure electron dot diagram of each ion is used to construct the Lewis Structure electron dot diagram for the ionic compound. Are all four hydrogen atoms in the same plane.
In almost all Fluorine and neon have seven and eight dots respectively. Magnesium has 2 valence electrons and Fluorine has 7 valence electrons in order for the 2 elements to combine you need 1 Mg atom and 2 F atoms The Mg gives one electrons to. Which Lewis electron-dot diagram represents a nitrogen atom in the ground state.
Which Lewis electron - dot diagram represents a fluoride ion. Draw a Lewis electron dot diagram for an atom or a monatomic ion. A C D B When an atom loses one or more electrons this atom becomes a A positive ion with a radius smaller than the radius of this atom B positive ion with a radius larger than the radius of this atom C negative ion with a radius smaller than the radius of this atom D negative ion with a radius larger than the radius.
The allene molecule has the following Lewis structure. Valence electrons in Oxygen 6. So Fluorine needs one electron to fill the Octet structure.
Start studying Lewis structure and vsepr. 35 Which Lewis electron dot diagram represents a. Draw the The following Lewis diagram represents the valence electron What is the Lewis Dot Diagram for Platinum.

What Is The Lewis Electron Dot Diagram For A Fluoride Ion
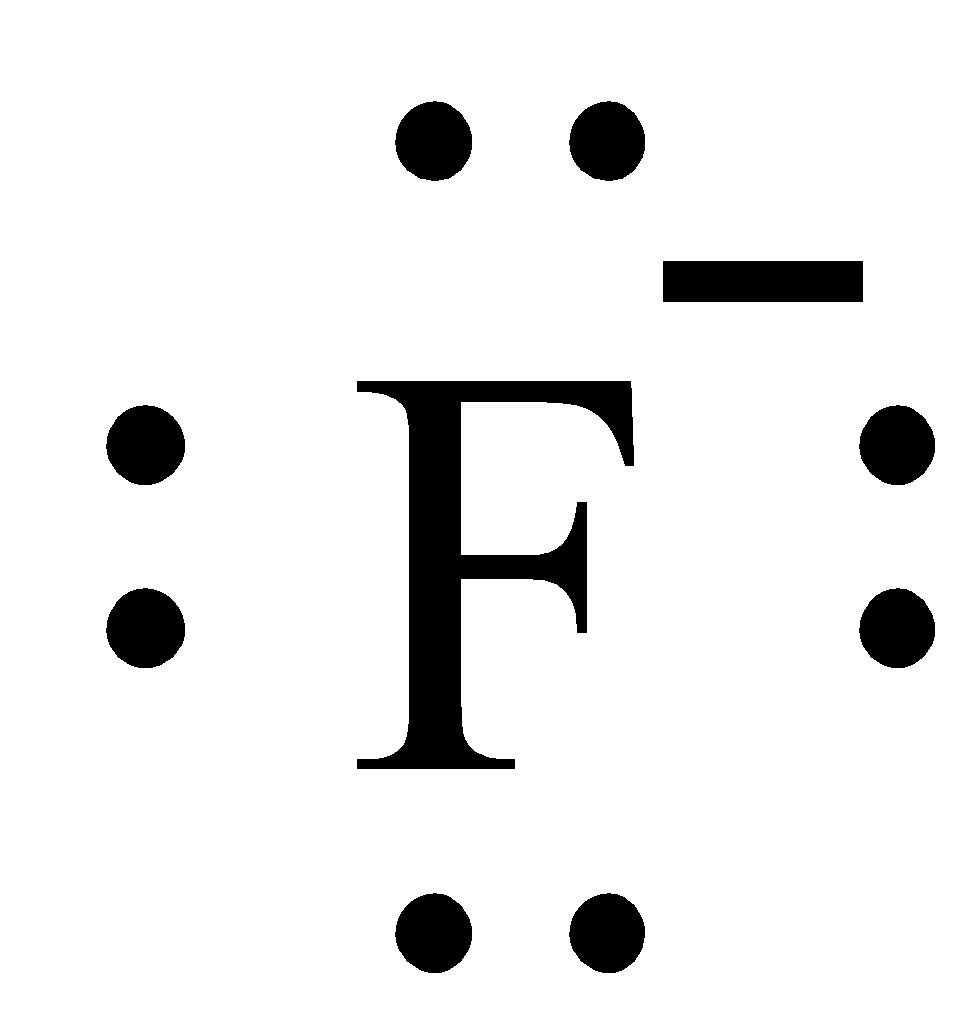
What Is The Lewis Electron Dot Diagram For Fluoride Class 11 Chemistry Cbse

How To Draw The Lewis Dot Structure For F Fluoride Ion Youtube
Comments
Post a Comment